Our Mission
Our vision is to enhance our global leadership position and grow with our customers as their preferred and trusted partner. We will achieve this by delivering top quality products, ensuring 100% reliable service, offering a larger product portfolio and newer solutions. With passion, perseverance and the support of our brilliant teams, we can change the face of nuclear medicine
Our Vision
For patients:To provide the necessary nuclear diagnostics and therapy that allow for better lives every day.For customers: To act as a trusted end-to-end solution provider, delivering high-quality products, unrivaled reliability and superior service. To go further, faster.For employees: To build on our remarkable culture and fulfill the passion, pride, ambition and future of all employees.
VISION, MISSION & VALUES
Our Vision
Our vision is to enhance our global leadership position and grow with our customers as their preferred and trusted partner. We will achieve this by delivering top quality products, ensuring 100% reliable service, offering a larger product portfolio and newer solutions. With passion, perseverance and the support of our brilliant teams, we can change the face of nuclear medicine
Our Mission
For patients:
To provide the necessary nuclear diagnostics and therapy that allow for better lives every day.
For customers:
To act as a trusted end-to-end solution provider, delivering high-quality products, unrivaled reliability and superior service. To go further, faster.
For employees:
To build on our remarkable culture and fulfill the passion, pride, ambition and future of all employees.
Our Management
In June 2021 IASON and ARGOS were acquired by Curium, and hence Benoit Woessmer and Xavier Defourt took over as new Managing Directors of IASON GmbH.
As of 1st May 2022, all PET business activities were transferred from IASON to ARGOS.
The management of Curium Austria GmbH consists of Benoit Woessmer and Xavier Defourt.
Our Culture
The name ‘Curium’ honors the legacy of pioneering radioactive researchers Marie and Pierre Curie, after whom the radioactive element curium was named.
Today, we build on their legacy with our life-saving diagnostics, reaching over 14 million patients every year. We use our unparalleled expertise to explore the untapped potential of nuclear medicine, providing quality products, quality of care and quality of life. We strive to transform each life we touch.

Our Guiding Principles
Production and delivery of our sensitive products meet highest quality standards, following national (e.g. AMBO 2009) and international regulations (e.g. GMP – Good Manufacturing Practice, GDP – Good Distribution Practice, ADR – transport of dangerous goods etc). Our employees are highly trained and following strict guidelines and internal procedures when carrying out their complex tasks. IASON is also certified according to the ISO 9001:2015 Management System.
Curium helps thousands
of patients afflicted
with cancer live
longer and better lives!
Our Management
In June 2021 IASON and ARGOS were acquired by Curium, and hence Benoit Woessmer and Xavier Defourt took over as new Managing Directors of IASON GmbH.
As of 1st May 2022, all PET business activities were transferred from IASON to ARGOS. Maximilian Hudl joined the company as the new operative Managing Director of ARGOS, so that - since 1st of May 2022 - the management of ARGOS Zyklotron Betriebs Ges.m.b.H consists of Benoit Woessmer, Xavier Defourt and Maximilian Hudl.
Our Culture
The name ‘Curium’ honors the legacy of pioneering radioactive researchers Marie and Pierre Curie, after whom the radioactive element curium was named.
Today, we build on their legacy with our life-saving diagnostics, reaching over 14 million patients every year. We use our unparalleled expertise to explore the untapped potential of nuclear medicine, providing quality products, quality of care and quality of life. We strive to transform each life we touch.
Competences
Radiopharmaceuticals
What is PET?
Process
IASON‘s core competence lies in the development, production and distribution of radioactive pharmaceuticals for application in Positron-Emission-Tomography (PET). Our USPs are clearly our Marketing Authorizations (MAs) which we hold for our products in several European countries (see /products).
Our products Efdege®, IASOcholine®, IASOflu®, IASOdopa® and IASOglio® are mainly used for PET, a technique used for producing high-resolution images of cancer, but application today also extends to a growing number of non-oncological indications, e.g. the detection of micro-fissures of the spine in case of back-pain of ambiguous origin. PET radiopharmaceuticals are considered to be consumables required for positron-emission-tomography.
New PET radiopharmaceuticals are constantly being developed into a marketable commodity in the R&D department of the company group. The company policy is to bring a new product to Marketing Authorization every two years. IASON is the only producer world-wide who has received Marketing Authorizations (which is equivalent to the American FDA approval) for six different radiopharmaceuticals for PET.
Positron-emission-tomography (PET) has become one of the most important medical diagnosis methods, especially for cancer patients. PET is a nuclear medicine imaging technique that produces a three-dimensional image or picture of functional processes in the body. Its most common use is to detect and depict tumors in cancer patients (oncological indication).
The principle of the examination is as follows: the (weakly) radioactive isotope fluor-18 is connected to glucose (sugar) and then injected into the patient’s body. The substance enters the blood circuit and is distributed within the whole body. It is then particularly those cells with a high metabolic activity (that is, cells which require a lot of sugar, like e.g. cancerous cells) which show a high uptake of this substance. This enrichment in turn is then made visible and can be seen as a ‘hot spot’ or ‘activity’ in the body.
The resulting images enable doctors to plan further treatment and decide whether to perform an operation, continue with chemotherapy or initiate radiation therapy. Pet examinations conducted with IASON’s products therefore help to improve the quality of the lives of cancer patients, because a great impact on the following levels of patient treatment has been scientifically proven:
- Change of initial therapy concept (e.g. from expensive chemotherapy to less costly radiation therapy)
- Proof of efficacy (e.g. of expensive chemotherapy, which will be stopped if efficacy is not proved by PET/CT)
- Preparing more efficient surgical intervention, based on highly precise information about the extension of a cancer
Production
IASON owns and operates two state-of-the-art radiopharmaceutical production sites in Austria (Linz and Klagenfurt). Further sites are continuously added to the IASON production network by means of Contract Manufacturing Organizations (CMOs) and License partners.
A cyclotron is at the heart of the complex production process, which meets highest standards which are defined by national (e.g. AMBO 2009) and international law (e.g. GMP Good Manufacturing Practice, GDP Good Distribution Practice, ADR transport of dangerous goods).
Delivery
Our pharmaceutical products have a half-life of only about 2 hours – therefore, no storage is possible and in-time logistics are crucial. Hence, our pharmaceuticals are produced during the nighttime and then immediately distributed to the customers – mostly public, but also private hospitals – by road and air logistics.
The patients are ready and prepared for the examinations when the products arrive at the hospitals. Usually, one delivery contains different products for several examinations (multi-vial).
Our PET radiopharmaceuticals are produced at the company-owned production sites in Linz and Klagenfurt at night and are delivered to the hospitals in the early morning hours.
These products only have a half-life of about two hours, which means that after this time, half of the product has already disappeared. This timely production and delivery to the hospital is one of the key competences of IASON.

Our Production Cycle
01

O-18 WATER
O-18 enriched water is one of the key starting materials required for the production process.
02
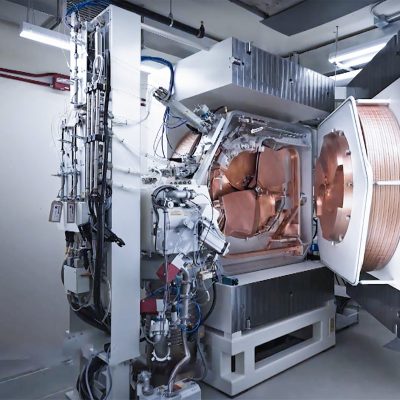
Cyclotron
A cyclotron (a small particle accelerator) is at the heart of the complex production process.
03

Tracer Synthesis
The tracers are synthesized by means of specialized dispensing systems.
04
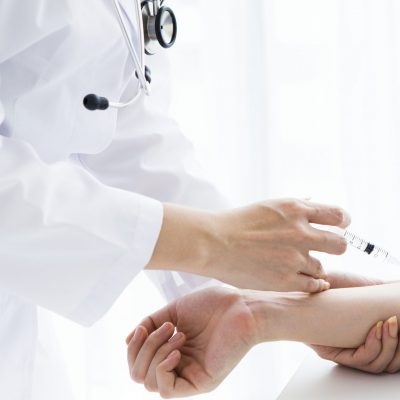
i. v. Injection
The finished tracers are delivered to the hospitals in the early morning hours, where the patients are already fully prepared for the PET Scanning.
01
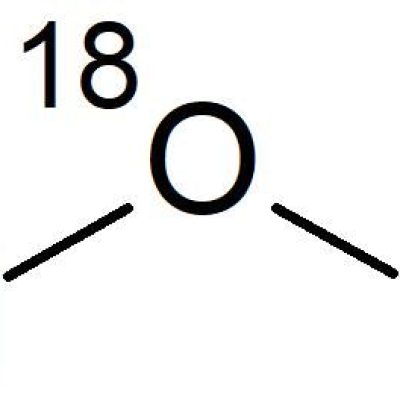
O-18 WATER
O-18 enriched water is one of the key starting materials required for the production process.
02

Cyclotron
A cyclotron (a small particle accelerator) is at the heart of the complex production process.
03

Tracer Synthesis
The tracers are synthesized by means of specialized dispensing systems.
05
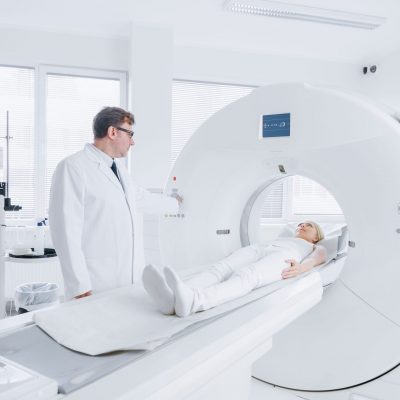
PET/CT Scanning
After the injection, the patient must wait a pre-defined period of time for the tracer to be distributed in the body before the PET scanning can begin.
06
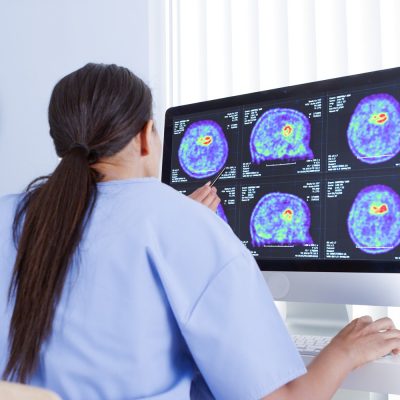
PET/CT Scan
The results of the PET scanning help doctors understand their patients’ condition and plan the next therapeutic steps.
04

i. v. Injection
The finished tracers are delivered to the hospitals in the early morning hours, where the patients are already fully prepared for the PET Scanning.
05

PET/CT Scanning
After the injection, the patient must wait a pre-defined period of time for the tracer to be distributed in the body before the PET scanning can begin.
06

PET/CT Scan
The results of the PET scanning help doctors understand their patients’ condition and plan the next therapeutic steps.